
Jun 14, 2024 | APSARD in the News, APSARD Members in the News, Uncategorized
On June 13th, 2024, the US Department of Justice announced a federal health care fraud indictment against a large subscription-based telehealth company that provides attention-deficit/hyperactivity disorder (ADHD) treatment to approximately 30,000-50,000 patients across the United States.
As per the US Department of Justice: “Ruthia He, the founder and CEO of Done Global Inc., was arrested in Los Angeles and David Brody, the clinical president of Done Health P.C., was arrested in San Rafael, California” for allegedly “developing and carrying out a $100 million scheme to defraud taxpayers and provide easy access to Adderall and other stimulants for no legitimate medical purpose.” As alleged in the indictment, “the defendants provided easy access to Adderall and other stimulants by exploiting telemedicine and spending millions on deceptive advertisements on social media. They generated over $100 million in revenue by arranging for the prescription of over 40 million pills.“
At the same time, the CDC issued the statement “Disrupted Access to Prescription Stimulant Medications Could Increase Risk of Injury and Overdose,“ which discusses the need for ongoing access to care and the potential disruption to as many as 30,000-50,000 patients ages 18 years and older across all 50 US states.
Within hours, the CDC hosted a meeting of the “ADHD Partners” to discuss these issues and the impact on the communities affected by ADHD. This meeting included Stephen Faraone from the World Federation for ADHD, CHADD leadership, the president of ADDA, ADHD coaches, other affiliated organizations, and me, from APSARD.
The overarching theme of this meeting was the need for holistic ADHD care, the pivotal role of APSARD and our affiliated ADHD organizations in decreasing stigma and improving equitable delivery of education and ADHD care.
I would like to share a resource which was just E-published in CNS Spectrums, ahead of print last week, which utilizes an “Expert Consensus Statement” on the strengths and limitations of telemedicine for ADHD management and care.
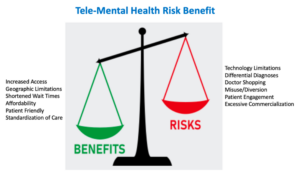
All in all, these updates highlight the pivotal role of APSARD as the leading voice for implementing ADHD science, education, and advocacy for individuals with ADHD.
Appreciatively,
Greg Mattingly MD
President, American Society for ADHD and Related Disorders
Supporting Links:
Founder/CEO and Clinical President of Digital Health Company Arrested for $100M Adderall Distribution and Health Care Fraud Scheme
Disrupted Access to Prescription Stimulant Medications Could Increase Risk of Injury and Overdose
Expert Consensus Statement for Telepsychiatry and Attention Deficit Hyperactivity Disorder
May 12, 2020 | APSARD Members in the News
APSARD and ADHD in Adults
APSARD loves getting to collaborate with likeminded organizations! Recently, Drs. Greg Mattingly and David Goodman, two APSARD Board members, spoke for two ADHD in Adults webinars. See the links below to check out both sessions:


Apr 27, 2020 | APSARD Members in the News
APSARD Members in the News
APSARD Board member and Membership Committee chair, Dr. Greg Mattingly, spoke about all things ADHD and Covid-19. Take a moment and check out these interviews!
Link to FOX 59 News interview
Link to Fox 19 Now interview
Link to Fox 31 Denver interview
Mar 9, 2020 | Annual Conference, APSARD Members in the News, Conference Blog, Editorial
Vallon Pharmaceuticals Presents Positive Data from Pilot Intranasal Human Abuse Study of Its Investigational Abuse Deterrent Stimulant, ADAIR, at the American Professional Society of ADHD and Related Disorders (APSARD) Annual Meeting
– According to reports from the US Department of Health and Human Services, more than 5 million Americans misuse or abuse prescription stimulants annually
– ADAIR is a novel, patented formulation of dextroamphetamine under development for the treatment of ADHD and narcolepsy that is designed to deter attempts to crush and snort it or take it by other non-oral routes that can produce a greater “high”
PHILADELPHIA–(BUSINESS WIRE)–Vallon Pharmaceuticals Inc., a specialty pharmaceutical company focused on the development of novel drugs for CNS disorders, today announced the presentation of positive data from a pilot study assessing human abuse liability for its investigational Abuse Deterrent Amphetamine Immediate Release (ADAIR). The data were presented this weekend at the 2020 American Professional Society of ADHD and Related Disorders (APSARD) Annual Meeting in Washington, D.C. ADAIR, the Company’s lead investigational new drug, is in development for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.
The poster, titled, “A Pilot Human Abuse Potential Study in Recreational Stimulant Drug Users Assessing Safety, Pharmacokinetics and Abuse Liability of Intranasally Administered Manipulated ADAIR and Dextroamphetamine Sulfate Tablets,” reported data from an intranasal (snorting) clinical trial of ADAIR, Vallon’s novel formulation of immediate release dextroamphetamine. The results of this 16-subject trial (VAL-103) demonstrated that intranasal administration of manipulated ADAIR was generally well tolerated, with all adverse events considered mild or moderate, and no new safety or tolerability signals identified. In this crossover comparative study, as compared to crushed and snorted dextroamphetamine sulfate, ADAIR demonstrated a blunted pharmacokinetic profile (lower Cmax, delayed Tmax, and lower AUC, especially during the early hours after administration). In addition, as compared to dextroamphetamine sulfate, even after extensive manipulation, ADAIR, when snorted, was less desirable to recreational drug abusers on key measures of abuse liability, in particular the Emax drug-liking scale (primary pharmacodynamic endpoint).
“Results from this pilot study suggest that Vallon’s investigational immediate release stimulant, ADAIR, may demonstrate less abuse potential than standard dextroamphetamine when manipulated and misused intranasally,” said Dr. Timothy Whitaker, a board-certified psychiatrist and Vallon’s Chief Medical Officer. “We appreciate the opportunity to present these findings to the attendees at APSARD, many of whom are at the forefront of ADHD research and patient care.”
According to reports from the US Department of Health and Human Services, more than 5 million Americans misuse or abuse prescription stimulants annually, most commonly teenagers and young adults. Separate studies report that approximately 40% of people who misuse prescription stimulants do so by snorting them.
“While we plan to consult with the FDA and conduct additional clinical trials, the data presented at APSARD, combined with market research feedback from physicians who treat ADHD and parents of teenagers and young adults who are prescribed ADHD stimulants, suggest that ADAIR, if approved, could be an important addition to available treatments for ADHD,” said David Baker, President & Chief Executive Officer of Vallon.
(more…)




